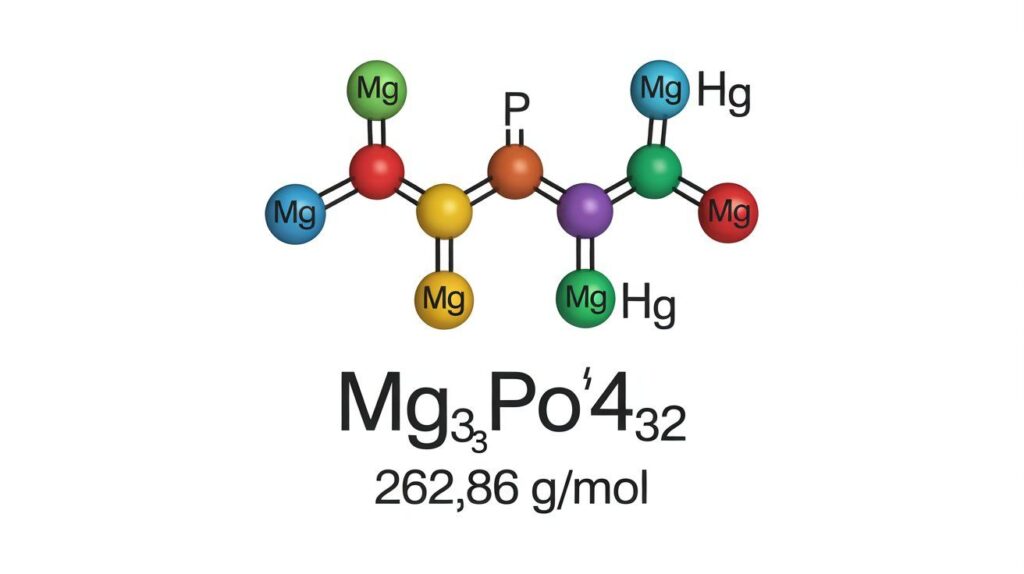
The molar mass of magnesium phosphate (Mg₃(PO₄)₂) is ~262.86 g/mol, essential for stoichiometry, chemical reactions, hydrates, and molecular weight calculations.
Molar mass of magnesium phosphate
The molar mass of magnesium phosphate (Mg₃(PO₄)₂) is about 262.86 g/mol. This value is critical in stoichiometry, chemical reactions, and laboratory experiments because it converts grams to moles accurately.
Understanding how magnesium phosphate’s molar mass is calculated also reveals variations caused by hydrates or isotopes, which may slightly change the molecular weight of magnesium phosphate.
What Is Magnesium Phosphate?
Magnesium phosphate (Mg₃(PO₄)₂) is an inorganic compound made of magnesium, phosphorus, and oxygen. It exists in several forms—mono, di, and tribasic—and is widely used in fertilizers, supplements, and food additives.
Knowing the molar mass of magnesium phosphate helps chemists calculate precise measurements in stoichiometry, industrial chemistry, and biological studies, ensuring accurate results across applications.
Chemical Formula and Atomic Masses
The formula for magnesium phosphate is Mg₃(PO₄)₂, containing 3 magnesium atoms, 2 phosphorus atoms, and 8 oxygen atoms.
Using standard atomic weights—Mg (24.305), P (30.974), and O (15.999)—we can calculate the molar mass of magnesium phosphate.
These values form the basis for precise molecular weight and percent composition analysis in chemistry.
Step-by-Step Calculation of Molar Mass of Magnesium Phosphate
To find the molar mass of magnesium phosphate (Mg₃(PO₄)₂), multiply atomic weights by their counts: 3 × 24.305 (Mg) = 72.915, 2 × 30.974 (P) = 61.948, 8 × 15.999 (O) = 127.992.
Adding these gives 262.86 g/mol, the precise molecular weight of Mg₃(PO₄)₂, essential for accurate stoichiometry.
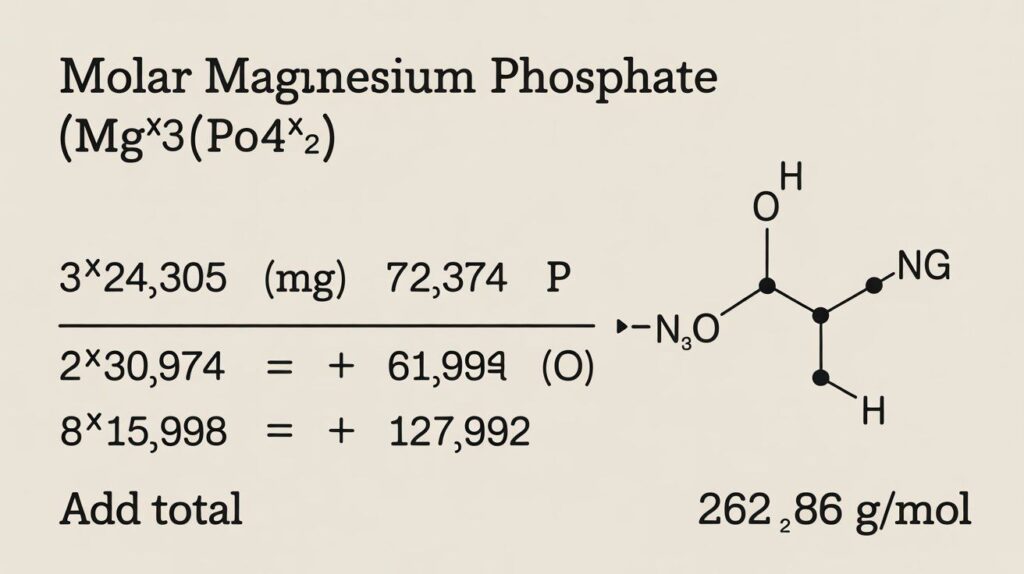
Worked Example and Verification
For example, 2 moles of magnesium phosphate weigh about 525.7 g (2 × 262.86 g/mol). This confirms the molar mass of magnesium phosphate (Mg₃(PO₄)₂) through conversion between moles and grams.
Published references list 262.85–262.87 g/mol, and such small differences arise from atomic weight rounding, not calculation errors.
Variations and Hydrates
| Variation Type | Formula | Effect on Molar Mass | Notes / Importance |
|---|---|---|---|
| Anhydrous | Mg₃(PO₄)₂ | 262.86 g/mol | Standard molar mass used in calculations |
| Hydrate | Mg₃(PO₄)₂·nH₂O | Increases based on n × 18.015 g/mol | Water molecules add to total mass; important in lab and industrial formulations |
| Isotopic Variation | Mg₃(PO₄)₂ | Slight increase/decrease | Different isotopes of Mg, P, or O alter molecular weight slightly |
| Combined Hydrate & Isotopic | Mg₃(PO₄)₂·nH₂O with isotopes | Variable | Important for precise stoichiometry and chemical reactions |
Why Knowing the Molar Mass Matters
Knowing the molar mass of magnesium phosphate (Mg₃(PO₄)₂) is crucial for accurate stoichiometry, chemical reactions, and reagent preparation.
It ensures precise measurements in laboratory experiments, industrial formulations, and supplements.
Accurate knowledge of the molecular weight of magnesium phosphate also helps in calculating percent composition and understanding variations caused by hydrates or isotopic differences.
Common Mistakes and Tips
Common errors in calculating the molar mass of magnesium phosphate (Mg₃(PO₄)₂) include forgetting coefficients, confusing molar mass with molecular weight, and rounding atomic weights too early.
To ensure accuracy, always double-check calculations, use the standard atomic weights, and carefully account for hydrates or isotopic variations when determining the molecular weight of magnesium phosphate.

FAQs
Q1: What is the exact molar mass of magnesium phosphate?
A: The molar mass of magnesium phosphate (Mg₃(PO₄)₂) is ~262.86 g/mol.
Q2: How do you calculate the molar mass of Mg₃(PO₄)₂ step by step?
A: Add the total atomic weights: (3 × 24.305) + (2 × 30.974) + (8 × 15.999) = 262.86 g/mol.
Q3: Why do some sources list 262.85 g/mol while others say 262.87 g/mol?
A: Small differences come from rounding atomic weights. Both are correct.
Q4: Does hydration change the molar mass?
A: Yes. Hydrated magnesium phosphate (e.g., Mg₃(PO₄)₂·8H₂O) includes water molecules, increasing the total molar mass.
Q5: How is molar mass different from molecular weight?
A: Both are often used interchangeably, but molar mass is in g/mol, while molecular weight is dimensionless.

Hamid Raza, aged 65, is a seasoned expert in nutrition, health supplements, and wellness, with over four decades of experience researching and educating people about essential minerals like magnesium. His work focuses on helping individuals improve energy, bone health, muscle function, and overall wellness through scientifically-backed magnesium knowledge.
Throughout his career, Hamid has contributed to numerous health articles, research studies, and wellness blogs, making complex nutritional science accessible to everyday readers. Passionate about natural health solutions, he guides readers on choosing the right magnesium supplements for optimal health.