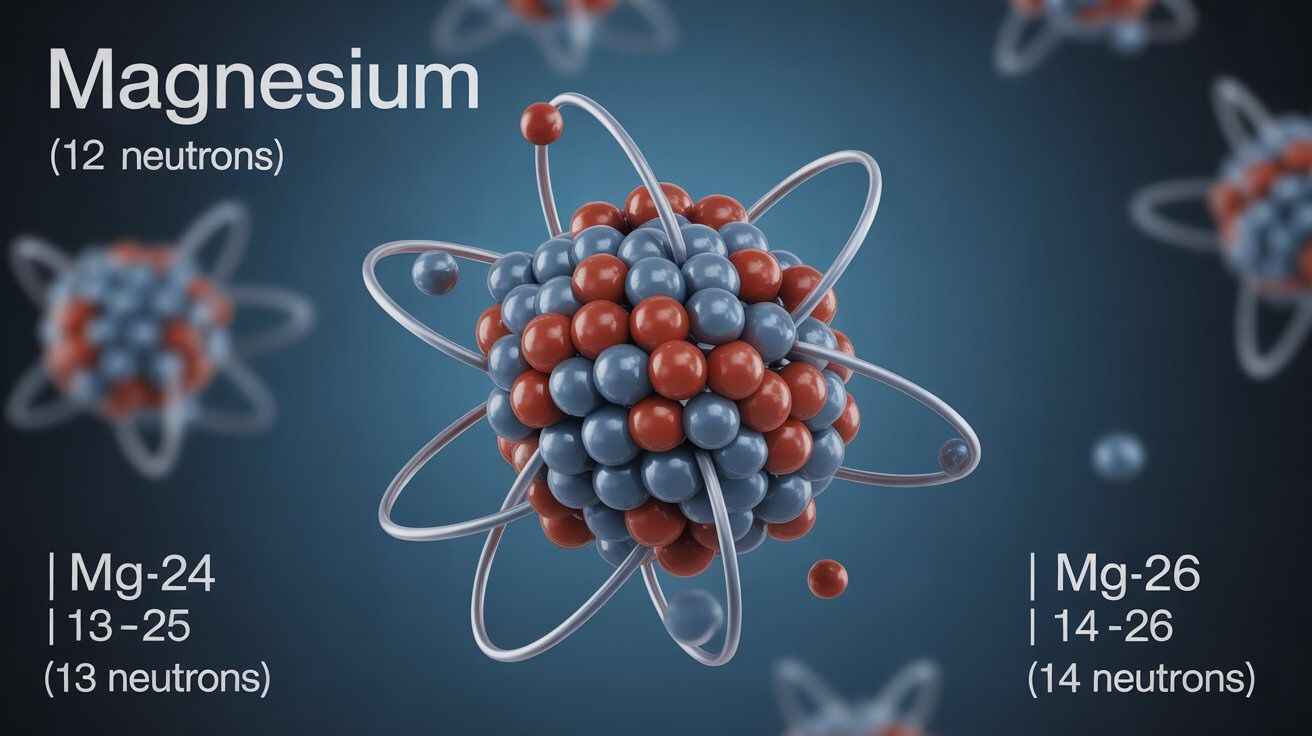
Number of neutrons for magnesium varies by isotope: Mg-24 → 12, Mg-25 → 13, Mg-26 → 14. Exotic forms like Mg-40 hold 28 neutrons.
Number of neutrons for magnesium
Magnesium has an atomic number of 12, meaning every atom carries 12 protons. The number of neutrons for magnesium varies with isotopes. Magnesium-24 contains 12 neutrons, magnesium-25 has 13, and magnesium-26 holds 14. These small differences in neutron count explain isotope stability, natural occurrence, and why magnesium is a useful case in atomic science.
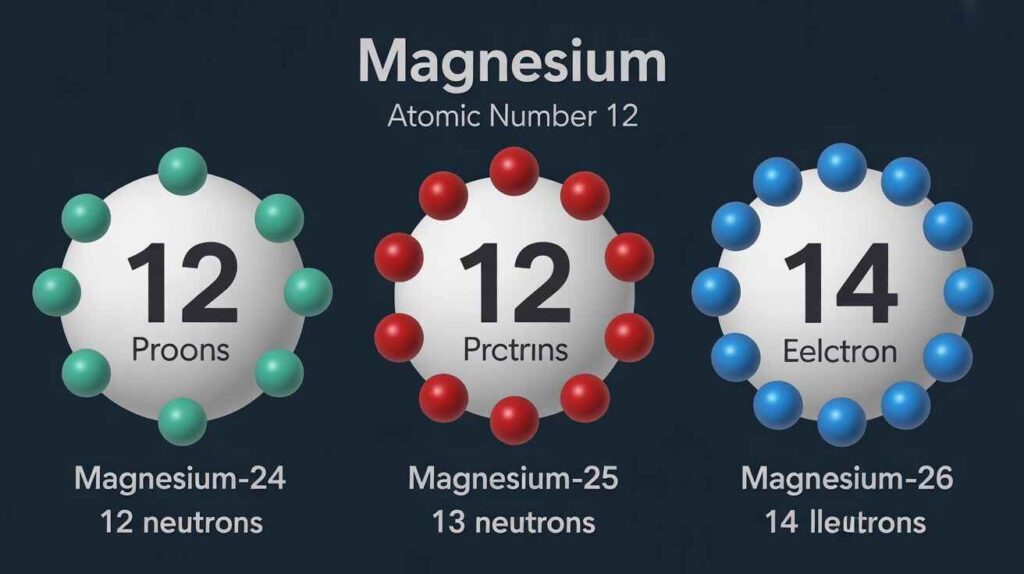
Understanding the Number of Neutrons for Magnesium
The number of neutrons for magnesium is calculated by subtracting its atomic number (12) from the isotope’s mass number. For example, Mg-24 → 24 – 12 = 12 neutrons. This rule applies to all isotopes. Neutrons stabilize the nucleus, balancing the repulsion between protons and shaping the unique properties of each magnesium isotope.
How to Calculate Neutrons in Magnesium
To find the number of neutrons for magnesium, use the formula: Neutrons = Mass Number – Atomic Number. Since magnesium’s atomic number is 12, simply subtract 12 from the isotope’s mass number. Example: Mg-25 → 25 – 12 = 13 neutrons. This method works for all isotopes, making calculations straightforward in chemistry and physics.
Magnesium Isotopes and Their Neutron Counts
| Isotope | Protons | Neutrons | Natural Abundance | Stability |
|---|---|---|---|---|
| Magnesium-24 | 12 | 12 neutrons | ~79% | Stable |
| Magnesium-25 | 12 | 13 neutrons | ~10% | Stable |
| Magnesium-26 | 12 | 14 neutrons | ~11% | Stable |
Exotic Neutron-Rich Isotopes of Magnesium
Beyond its stable forms, scientists have identified exotic isotopes such as magnesium-40 with 28 neutrons. These lie near the neutron drip line, where nuclei become unstable. Extra neutrons test nuclear stability and reveal how forces hold the nucleus together. Such discoveries expand our knowledge of atomic structure and the extreme limits of matter.
Why the Number of Neutrons Matters
The number of neutrons for magnesium influences nuclear stability and isotope behavior. Balanced neutron-to-proton ratios keep atoms stable, while excess or deficit leads to radioactivity. Neutrons also reduce proton repulsion inside the nucleus, allowing elements like magnesium to exist naturally. This balance explains isotope abundance and their importance in research, medicine, and geochemistry.
FAQs
Q1: How many neutrons does magnesium-24 have?
Magnesium-24 has 12 neutrons. It contains 12 protons (atomic number) and a mass number of 24, giving 24 – 12 = 12.
Q2: What is the neutron count of magnesium-25?
Magnesium-25 has 13 neutrons. With 12 protons and a mass number of 25, subtracting gives 25 – 12 = 13.
Q3: Why does the number of neutrons for magnesium vary?
Different isotopes have different mass numbers. Protons stay the same (12), but neutrons adjust to balance stability.
Q4: How do you calculate the number of neutrons?
Subtract the atomic number (12) from the isotope’s mass number. Example: Mg-26 → 26 – 12 = 14 neutrons.
Q5: What is the heaviest known isotope of magnesium?
Magnesium-40 is the heaviest known isotope, with 28 neutrons. It lies near the neutron drip line and is unstable.

Hamid Raza, aged 65, is a seasoned expert in nutrition, health supplements, and wellness, with over four decades of experience researching and educating people about essential minerals like magnesium. His work focuses on helping individuals improve energy, bone health, muscle function, and overall wellness through scientifically-backed magnesium knowledge.
Throughout his career, Hamid has contributed to numerous health articles, research studies, and wellness blogs, making complex nutritional science accessible to everyday readers. Passionate about natural health solutions, he guides readers on choosing the right magnesium supplements for optimal health.